Page 99 - 14_Atti_SIN_SNO_2022_flip
P. 99
Proceedings SNO “Percorsi clinici in Neuroscienze”
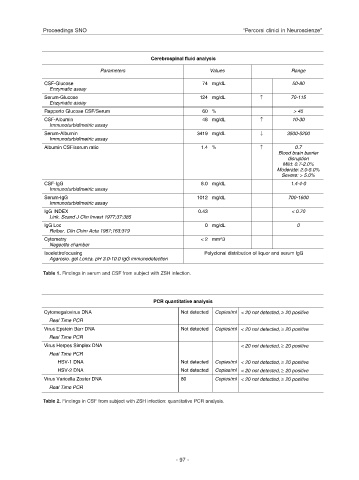
Cerebrospinal fluid analysis
Parameters Values Range
CSF-Glucose 74 mg/dL 50-80
Enzymatic assay
Serum-Glucose 124 mg/dL ↑ 70-115
Enzymatic assay
Rapporto Glucose CSF/Serum 60 % > 45
CSF-Albumin 48 mg/dL ↑ 10-30
Immunoturbidimetric assay
Serum-Albumin 3419 mg/dL ↓ 3500-5200
Immunoturbidimetric assay
Albumin CSF/serum ratio 1.4 % ↑ 0.7
Blood brain barrier
disruption
Mild: 0.7-2.0%
Moderate: 2.0-5.0%
Severe: > 5.0%
CSF-IgG 6.0 mg/dL 1.4-4-0
Immunoturbidimetric assay
Serum-IgG 1012 mg/dL 700-1600
Immunoturbidimetric assay
IgG INDEX 0.43 < 0.70
Link. Scand J Clin Invest 1977;37:385
IgG Loc 0 mg/dL 0
Reiber. Clin Chim Acta 1987;163:319
Cytometry < 2 mm^3
Negeotte chamber
Isoelettrofocusing Polyclonal distribution of liquor and serum IgG
Agarosio. gel Lonza. pH 3.0-10.0 IgG immunodetection
Table 1. Findings in serum and CSF from subject with ZSH infection.
PCR quantitative analysis
Cytomegalovirus DNA Not detected Copies/ml < 20 not detected, ≥ 20 positive
Real Time PCR
Virus Epstein Barr DNA Not detected Copies/ml < 20 not detected, ≥ 20 positive
Real Time PCR
Virus Herpes Simplex DNA < 20 not detected, ≥ 20 positive
Real Time PCR
HSV-1 DNA Not detected Copies/ml < 20 not detected, ≥ 20 positive
HSV-2 DNA Not detected Copies/ml < 20 not detected, ≥ 20 positive
Virus Varicella Zoster DNA 80 Copies/ml < 20 not detected, ≥ 20 positive
Real Time PCR
Table 2. Findings in CSF from subject with ZSH infection: quantitative PCR analysis.
- 97 -